126 years ago, on November 8, 1895, Wilhelm Conrad Röntgen discovered the x-rays, which in German carry his name. He was not the first to observe this new type of radiation, but he was the first to realize their potential. Today, x-rays are used not only in medicine as a diagnostic tool, but also in various fields of physics, such as crystal structure analysis or x-ray telescopes.
How are x-rays generated?
X-rays, like light, can be described as either an electromagnetic wave or a particle, called photon. The wavelength of x-rays is about ten to 1.000 times smaller than that of visible light (red: 800 nanometers to violet: 400 nanometers). This is a few picometers, which corresponds to one millionth of the width of a human hair. Since wavelength and energy are indirectly proportional to each other, the energy of x-rays is ten to 1.000 times greater than that of visible light.
To generate x-rays, an x-ray tube is normally employed. This tube consists of a glass container with a filament in it - similar to a light bulb - through which electrons flow (this means, a current). An applied high voltage accelerates the electrons from the filament toward a thick metal plate. In other words, the voltage gives the electrons the necessary energy to get out of the filament as they are strongly attracted by the metal plate.
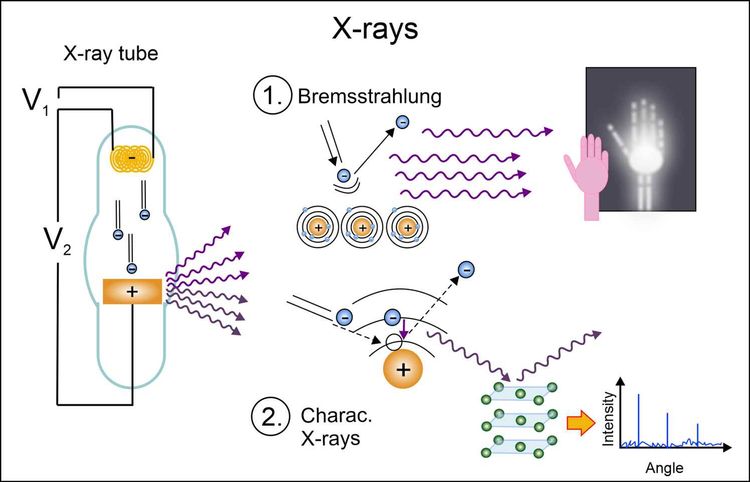
There are two mechanisms for the generation of x-rays, which are shown schematically in the figure below. In the first, the electrons are slowed down and deflected by the proximity of the atomic nuclei in the metal. In the process, they lose energy, which is emitted to the environment in the form of x-ray radiation (Bremsstrahlung). This type of radiation is used in medicine. In the second, the accelerated electrons can also kick out other electrons of the inner atomic shells of the metal¹. This causes places in the shells with low energy to become vacant and electrons from outer shells to fall down into these empty places. The additional energy that these electrons from higher shells possess is emitted in the form of characteristic x-rays as they fall down. This is used in materials research because it has very specific (discrete) energy values.
X-rays in the human body
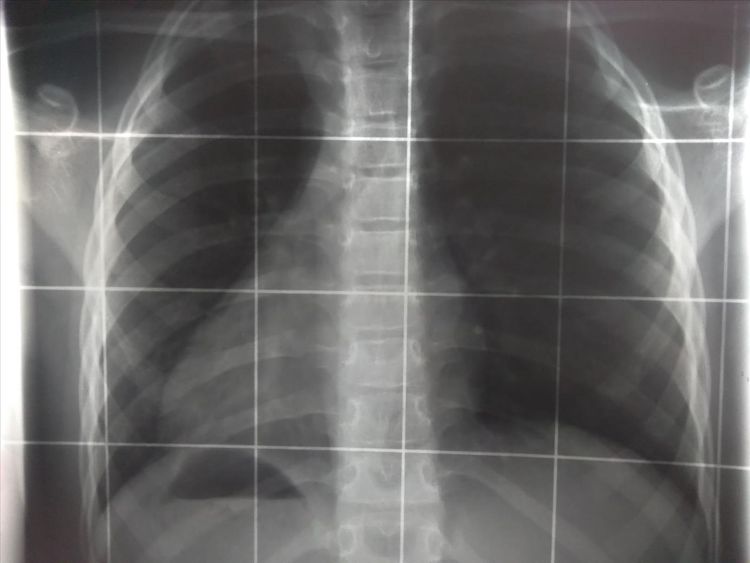
When we go to the dentist or to the radiology institute and an x-ray is taken, our body absorbs x-rays. The intensity of the rays then decreases exponentially with the length traveled in the material. This means: the deeper, the less the x-rays penetrate.
The materials from which our body is made, play also an important role: bones, tissue, blood, et cetera. This is because these materials have different absorption coefficients, which determine how well the radiation is absorbed and how deeply it penetrates into our body. This coefficient is greater the larger the atomic nucleus. For example: oxygen (which makes up most of our tissues) has a nucleus with eight protons and eight neutrons, while calcium (an important component of our bones) has a nucleus with twenty protons and twenty neutrons. Thus, the calcium in our bones absorbs x-rays better than the tissue around it. This effect is exploited when an x-ray image is taken, because it maps the intensity of the absorbed radiation in the body. Places where a lot of radiation arrives are dark, whereas places where hardly any radiation arrives are light. As a result, the bones are imaged in an intensity image with a high contrast to the surrounding tissue.
However, the contrast between different types of tissue is not as high, so tendon or ligament injuries, for example, cannot be diagnosed by a conventional x-ray image. Modern computer tomography, which mathematical basis was established by Austrian physicist Johannes Radon, can produce not only individual two-dimensional images, but an entire collection of two-dimensional images that allow a spatial reconstruction of a body part slice by slice, making diagnosis easier. Impressive computer tomography images in 3D can be viewed in the new anatomy room MED Space at Johannes Kepler University in Linz.
X-rays in solid state materials
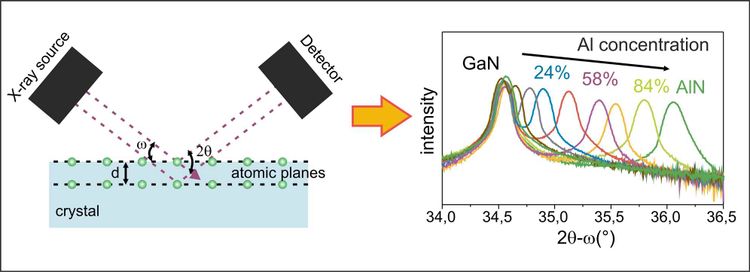
X-ray analyses are also standard in the crystal structure analysis of solids. The most common method is x-ray diffraction. For this purpose, it is exploited that the wavelength is of the same order of magnitude as the atomic distances in the crystal, which is a condition for diffraction². As shown schematically in the picture below, the rays fall on the sample at a previously selected angle and are diffracted, or deflected, by the individual atoms of the material under investigation - or rather by the electron clouds around the atomic nucleus. The image recorded at the detector shows an interference pattern: when the diffracted beams overlap, they amplify (maxima) or they cancel each other out (minima). By changing the angle of incidence and detection angle during the measurement, a pattern characteristic for the respective crystal is created. The diffraction pattern can be used to determine crystal atom spacings, densities of defects, material concentrations and strains in the crystal. An example of such a diffraction pattern is shown in the picture, where the shift of the peak maximum due to the addition of aluminum in a gallium nitride crystal is shown: The higher the aluminum concentration in the crystal, the larger the diffraction angle.
For x-ray diffraction, one wavelength is usually sufficient, whereas for other methods, such as x-ray absorption spectroscopy, the wavelength of the radiation must be varied. Since a normal x-ray tube produces only one characteristic wavelength, this experiment requires a visit to the synchrotron. Because of the various wavelengths, this method can be used to look at the absorption of different atoms in the crystal instead of diffraction, and an image characteristic of the particular material is recorded. Through this analysis, the exact positions of foreign atoms in the crystal can be obtained.
So, whether in medicine or in physics, x-rays are one of the remarkable discoveries of mankind, which usefulness and diversity will support our daily life, not only in science, for years to come. (Andrea Navarro-Quezada, Anna Spindlberger, 8.11.2021)
Fußnoten
¹ Every atom consists of a nucleus and several electron shells, where the electron shells very close to the nucleus have the lowest energy and are therefore more often filled with electrons than shells further away from the nucleus.
² Diffraction in physics is the deflection of a wave off an obstacle, which is sometimes why we can hear sound around corners. There is a vivid explanation here. Reflection, on the other hand, is the throwing back of the wave from an obstacle (for example, a mirror).
More blog posts